Lead-acid batteries store energy using a reversible chemical reaction between lead plates and dilute sulphuric acid (electrolyte). There are three basic types of lead acid battery – starter batteries: used to start engines in cars etc, deep-cycle batteries: used in renewable energy applications and camping etc, and marine batteries: used both for starting and for deep cycle applications.
Different Types of Lead Acid Battery
Starter batteries have many thin lead plates which enables them to discharge a lot of energy very quickly – i.e. to start a vehicle. However, if a starter battery is discharged deeply (more than 20-25% depth of charge), its plates can be permanently damaged and the lifetime of the battery greatly reduced.
Deep cycle batteries have fewer thicker lead plates, and so cannot discharge energy so quickly, but can be cycled deeply and recharged many times without damaging the battery. Deep cycle batteries are designed to provide a steady current over a long period of time.
Batteries and Renewable Energy
In renewable energy systems multiple batteries are usually connected together to make a battery bank. Click here to find out the correct way to connect batteries into a battery bank. Ideally a deep cycle battery should not be discharged below 40% charge, and should be kept fully charged whenever possible in order to maximise its useful lifetime. When selecting a battery (or battery bank) for a renewable system, this should be considered.
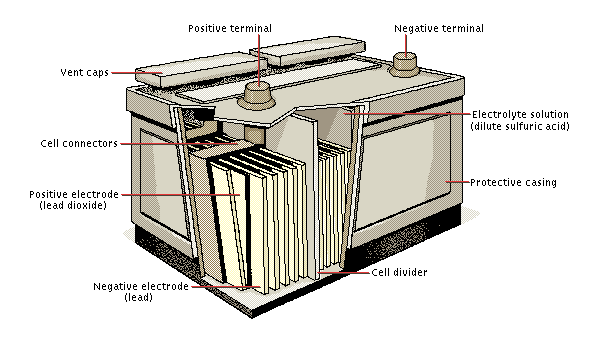
Inside a Lead Acid Battery
A 12 volt lead acid battery is actually made up of six identical 2 volt cells. Each cell contains lead plates of different compositions sitting in dilute sulphuric acid. Lead dioxide plates (linked to the positive terminal of the battery) react with the acid to form lead sulphate giving up electrons (leaving the plate positive). The pure lead plates (linked to the negative terminal of the battery) react with the sulphate ions to also form lead sulphate. The pure lead plates therefore supply two positive charges and so are left negative. The passage of electons from the lead oxide plates to the pure lead plates is the current of electricity generated by the cell which can be used.
When the battery is recharged, the lead sulphate in each cell is broken down resulting in lead dioxide being redeposited on the positive electrode, and lead being replaced on the negative electrode.
Lead acid battery schematic diagram follows with more information below.
 When batteries are frequently deeply discharged then sulphation build up occurs. Sulphur molecules from the battery acid (electrolyte) start to coat the lead of the plates. Once the lead is covered in sulphur the battery is dead and cannot be recharged. Sulphation starts occurring once the charge of a starting battery descends below 75%. Therefore lead acid batteries must be looked after well if they are to remain useable for a long time. Click here to read our article on battery desulphation – a method to bring dead batteries back to life.
When batteries are frequently deeply discharged then sulphation build up occurs. Sulphur molecules from the battery acid (electrolyte) start to coat the lead of the plates. Once the lead is covered in sulphur the battery is dead and cannot be recharged. Sulphation starts occurring once the charge of a starting battery descends below 75%. Therefore lead acid batteries must be looked after well if they are to remain useable for a long time. Click here to read our article on battery desulphation – a method to bring dead batteries back to life.Monitoring Lead Acid Batteries
Click here to find out how to make a Simply Battery Status Monitor to help you to look after the batteries in your renewable energy system.
Buying Lead Acid Batteries
Lead acid batteries are available in a wide range of voltages (typically 4, 6, 12, and 24 Volt), and capacities (from <1Ah up to many hundreds of Ah). For small 12V batteries of 1-20Ah of the type typically used in small solar powered projects (such as shed lighting) click here to view our article: 12 Volt Deep Cycle Batteries for Solar. For larger batteries, our article Deep Cycle Batteries for Sale may be helpful to you.